Home > Help > Faqs
Frequently Asked Questions (FAQ)
-
1. Why must my protocols be submitted?
All institutions and appliance are required to comply with certain regulations regarding studies in research activities within Ministry of Health.
-
2.When do I need to submit an application?
The research applicant can immediately submit his application through the website with all documents required.
-
3.What is the aim of Research Technical Support Team?
The aim of the RTST is to ensure that high ethical standards are maintained in research projects to protect the interests of research participants, investigators and the MOH.
-
4.How can I know the right committee to submit my research?
Research applicants must direct their applications to the relevant MOH Research Committees according to the following guidelines
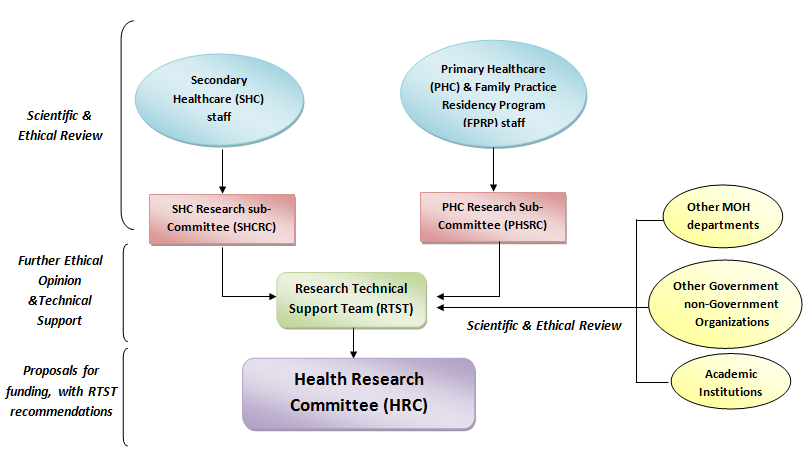
-
5.Who can answer questions about the protocol?
You may discuss your proposed project with the Research Office, or the members associated with your college or department.
-
6.When are the RTST meetings?
Meetings for the RTST are held once a week scheduled on every Monday depending on the number of submissions.
-
7.How long will the review process take?
The review processes time will vary based on several factors. The quality of the application, complexity of research methods, risk to participants, and the load of protocols pending review can all lead to longer approval times.
All complete applications will receive an initial response within two weeks of receipt by the Research Office.
-
8.How will researchers be notified of the status of their protocols?
An email will be sent to the principal investigator and the student researchers, if appropriate, regarding the status of the protocol after it has been reviewed initially.
-
9.What types of attachments should I include in my application?
- o A signed covering letter from the principal investigator to the chairperson of the relevant research committee (with the signature of the Department Head/Chairperson/head of the research body).
- o Completed, signed and dated Research Application Form.
- o Curriculum vitae of the Principal Investigator (applicant)and co-investigator(s)
- o Detailed Research proposal
- o Participant recruitment material: advertisements, information letters
- o Informed consent form on the principal investigator’s/Institution’s letterhead (Arabic and English).
- o Data collection tools (e.g. questionnaires, data collection forms, etc.)
- o Number of centres and sample size for each centre (for multi-centre studies)
- o Project timeline
-
• For student research, please also include:
- o Supporting letter from the Research Supervisor/Administration-in-charge/Head of the research body (on official letterhead).
- o Approval letter from the Ethics Review Board of the institution (on official letterhead).
-
10.Are there any requirements for an informed consent form?
Yes. All informed consent documents must include:
- • A description of the study itself and the study population
- • Approximately how long participating in the activity will take
- • How the data be used
- • A statement that the activity is voluntary and that subjects may withdraw at any time
- • A statement of the risks and benefits of participation
- • The Research Compliance Office contact information
- • A statement confirming that the individual gives their consent to participate
- • A signature line
-
11.Can I change the questionnaire after getting HRP approval?
No changes allowed after Approval, the committee has the authority to judge the applicant in case of any changes.
-
12.My project has been approved. Is there anything else that I need to do?
Once a project has been approved must seek permission and follow procedures as dictated by the concerned departments after presenting them with a valid MOH approval letter.
-
13.I am an undergraduate or graduate student. May I submit an application for a project?
All undergraduate students will be communicated with Ministry of Health research committee through their Faculty Advisor.
-
14.The application does not open on my computer. Can you send me a working copy?
All application forms found on the MOH website have been tested and verified to work across multiple platforms. Forms are dynamic. pdf files which will require Adobe Reader to operate properly. The following steps should allow you to open the forms and save your progress.
-
•
The forms have not been tested nor verified on tablets or smartphones
- Use a computer to complete the forms - • Download the newest version of the free Adobe Reader software
- • Save the application form you wish to use to your computer in a location that you will easily locate it
- • Open the Adobe Reader software
- • pen the form file from within Adobe Reader.
If you still have difficulty using the form, please contact the Research Office.
-
•
The forms have not been tested nor verified on tablets or smartphones
-
15.I am collaborating with researchers from another institution. The study has already been approved by another Committee. Do I still need to submit to the HRP?
Each collaboration is different, and other institutions each have unique requirements. Please check with the Research Office to discuss your circumstance.
-
16.Still I have questions?
You can contact the committee office through the following:
- Email: RTST@health.gov.bh
- Telephone: 17 28 6052 – 17 28 6050